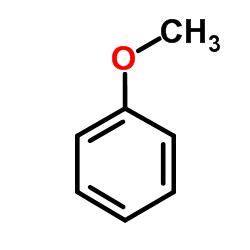
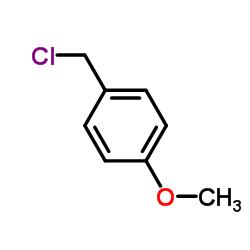
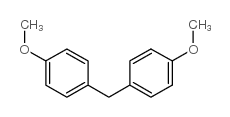
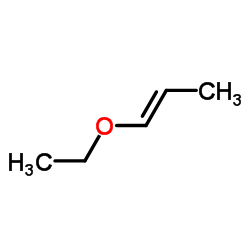
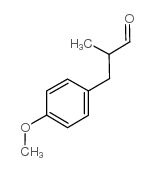
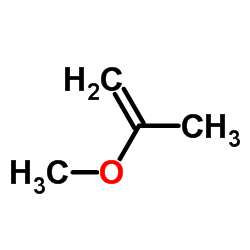
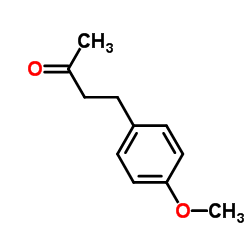
|
~% |
|
~% |
|
~30% |
|
~60% |
|
~69% |
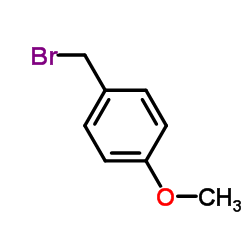
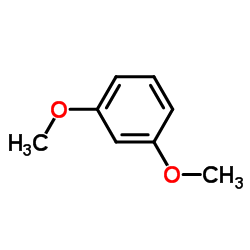
![2,4-dimethoxy-1-[(4-methoxyphenyl)methyl]benzene Structure](https://image.chemsrc.com/caspic/295/53039-53-5.png)
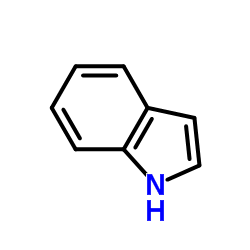

![3-[(4-methoxyphenyl)methyl]-1H-indole Structure](https://image.chemsrc.com/caspic/287/22044-95-7.png)