|
~% |
|
~% |
|
~% |
|
~22% |
|
~99% |
|
~96% |
|
~79% |
|
~97% |
|
~% |
|
~61% |
|
~% |
|
~30% |
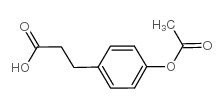

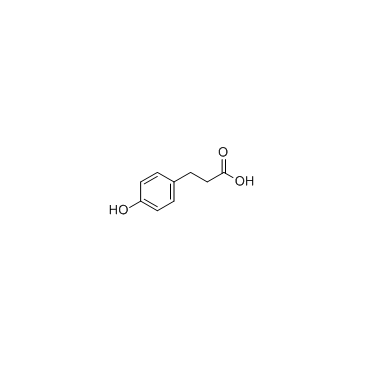

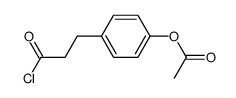
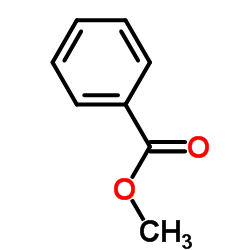

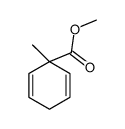

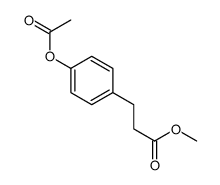
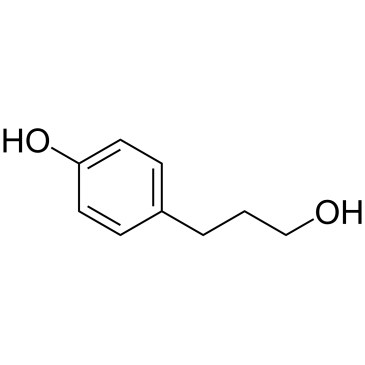
![8-(3-hydroxypropyl)-1,4-dioxaspiro[4.5]decan-8-ol Structure](https://image.chemsrc.com/caspic/473/90214-47-4.png)
![4,9,12-trioxadispiro[4.2.48.25]tetradecane Structure](https://image.chemsrc.com/caspic/128/65682-31-7.png)
![4,9,12-trioxadispiro[4.2.48.25]tetradeca-6,13-diene Structure](https://image.chemsrc.com/caspic/180/581784-88-5.png)
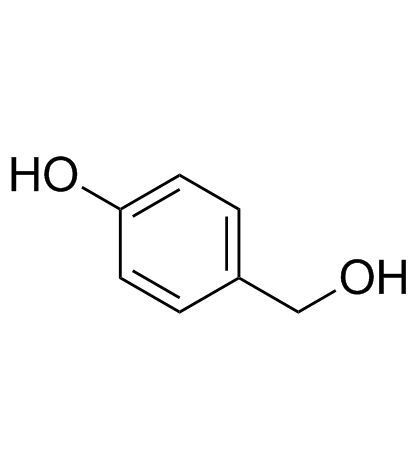
![1-oxaspiro[2.5]octa-4,7-dien-6-one Structure](https://image.chemsrc.com/caspic/247/54337-43-8.png)