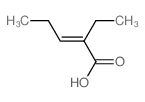
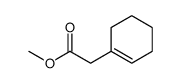
|
~97% |
|
~% |
|
~% |
|
~78% |
|
~77% |
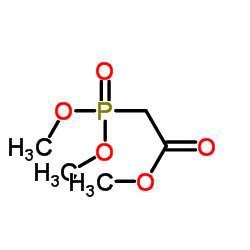

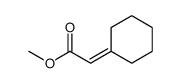
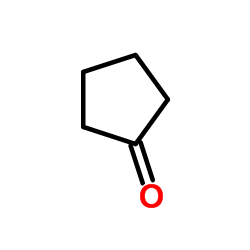
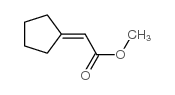
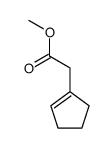
![2-[1-PHENYL-METH-(E)-YLIDENE]-SUCCINIC ACID DIETHYL ESTER Structure](https://image.chemsrc.com/caspic/457/23360-64-7.png)