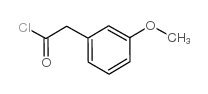
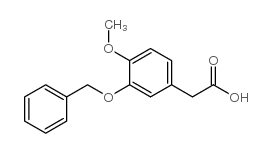
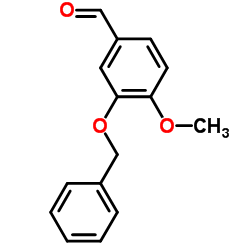
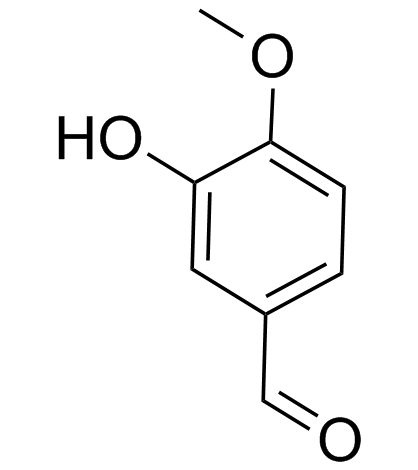
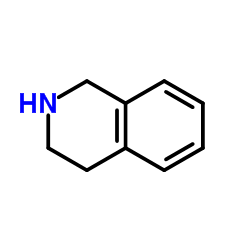
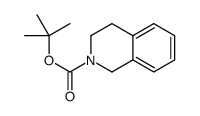
|
~68% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~30% |
|
~94% |
|
~% |
|
~% |
|
~98% |
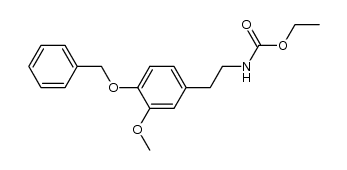
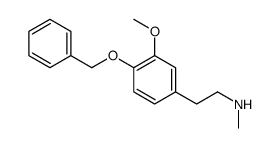
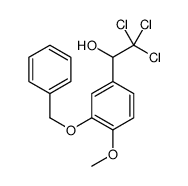
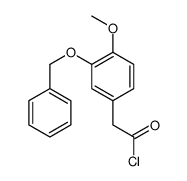
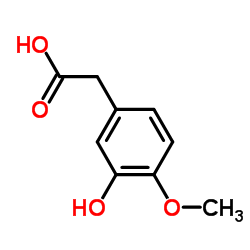
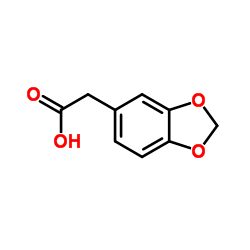
![BENZO[1,3]DIOXOL-5-YL-ACETYL CHLORIDE Structure](https://image.chemsrc.com/caspic/194/6845-81-4.png)