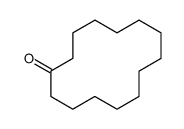
|
~% |
|
~4% |
|
~% |
|
~% |
|
~19% |
|
~16% |
|
~16% |
|
~% |
|
~23% |
|
~% |
|
~5% |
|
~% |
|
~9% |
|
~16% |
|
~36% |
|
~53% |
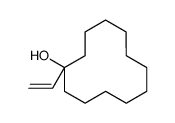
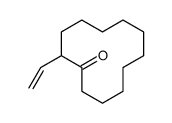
![2-(iodomethyl)-1-oxaspiro[2.5]octane Structure](https://image.chemsrc.com/caspic/202/139367-26-3.png)
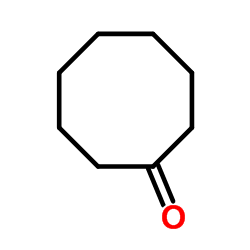
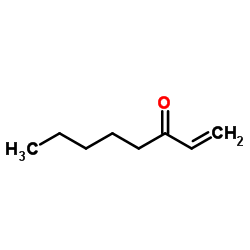
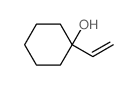
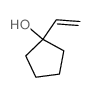
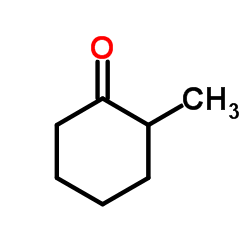

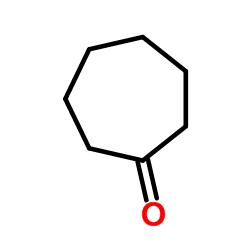

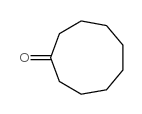

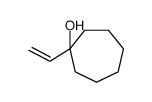
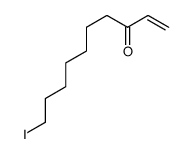
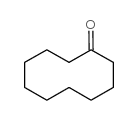
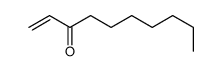
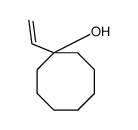
![2-(iodomethyl)-1-oxaspiro[2.7]decane Structure](https://image.chemsrc.com/caspic/126/139367-28-5.png)