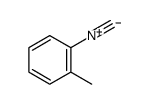
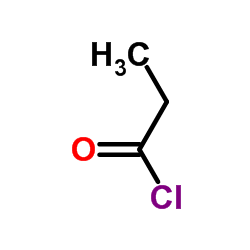
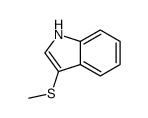
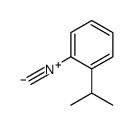
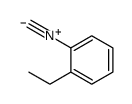
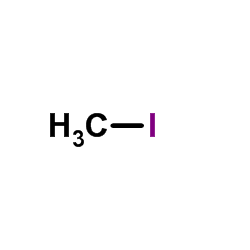
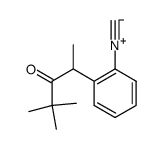
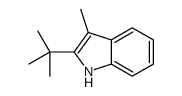
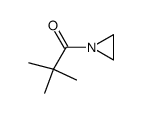
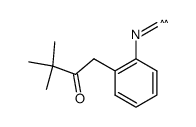
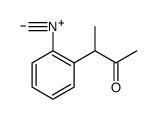
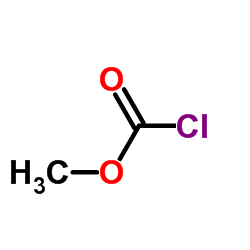
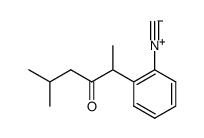
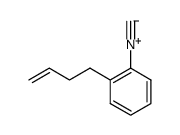
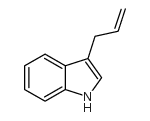
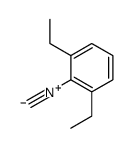
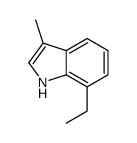
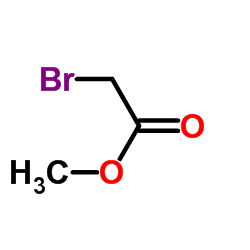
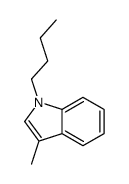
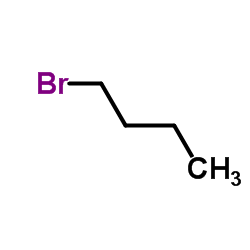
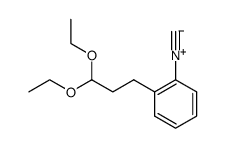
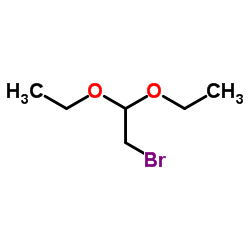
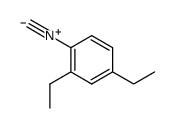
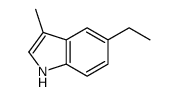
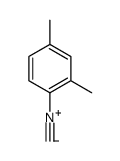
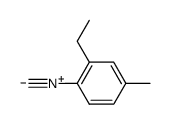
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~77% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |