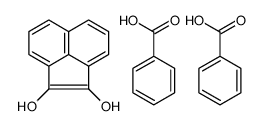
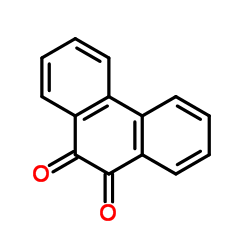
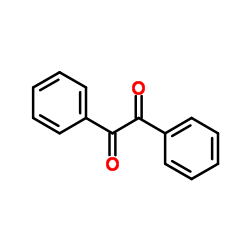
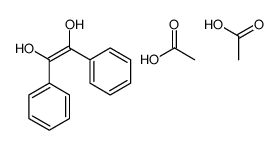
|
~72% |
|
~90% |
|
~98% |
|
~85% |
|
~92% |