|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~% |
|
~46% |
|
~% |
|
~99% |
|
~% |
|
~49% |
|
~17% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
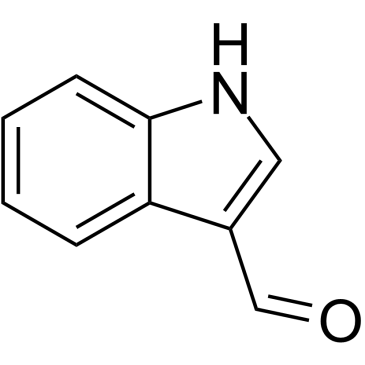
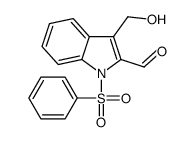
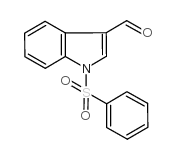
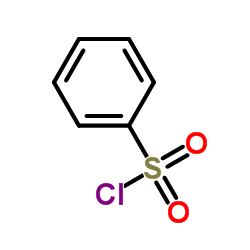

![[1-(PHENYLSULFONYL)-1H-INDOL-3-YL]METHANOL Structure](https://image.chemsrc.com/caspic/158/89241-33-8.png)
![1-[3-methyl-1-(phenylsulfonyl)indol-2-yl]ethanol Structure](https://image.chemsrc.com/caspic/383/143774-62-3.png)
![1-[1-(benzenesulfonyl)-3-methylindol-2-yl]ethanone Structure](https://image.chemsrc.com/caspic/345/143774-63-4.png)
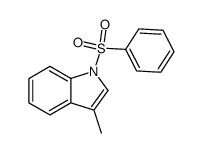
![4-(benzenesulfonyl)furo[3,4-b]indole Structure](https://image.chemsrc.com/caspic/496/89241-37-2.png)

![4-(benzenesulfonyl)-3-phenylfuro[3,4-b]indole Structure](https://image.chemsrc.com/caspic/090/143774-52-1.png)


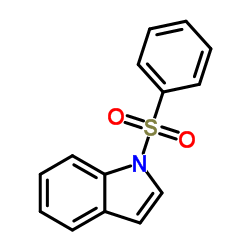
![[1-(PHENYLSULFONYL)-1H-INDOL-2-YL]METHANOL Structure](https://image.chemsrc.com/caspic/424/73282-11-8.png)
![[3-methyl-1-(phenylsulfonyl)indol-2-yl]phenylmethanol Structure](https://image.chemsrc.com/caspic/231/143774-66-7.png)