Biomimetic Cannabinoid Synthesis Revisited: Batch and Flow All-Catalytic Synthesis of (±)-ortho-Tetrahydrocannabinols and Analogues from Natural Feedstocks
Pascal D. Giorgi, Virginie Liautard, Mathieu Pucheault, Sylvain Antoniotti
Index: 10.1002/ejoc.201800064
Full Text: HTML
Abstract
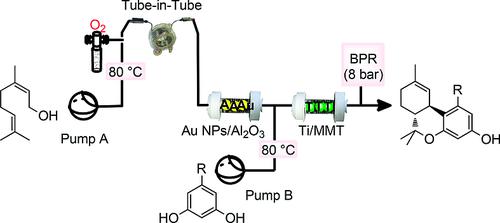
Using a combination of Au nanoparticle-catalyzed oxidation under an O2 atmosphere and a Ti-doped montmorillonite (Ti-MMT)-catalyzed tandem arylation/double cyclization, we developed an original and highly selective method for the synthesis of ortho-tetrahydrocannabinol derivatives from simple substrates. The reaction sequence could be performed in two steps in batch mode or in a single operation in continuous-flow reactors. The abnormal regioselectivity was proposed to be the result of the non-innocent role of the MMT support, TiIV cation coordination, and a Lewis acid-assisted Brønsted acid (LBA) mechanism. A short synthesis of (±)-tetrahydrocannabinol derivatives is described. This method relies on the oxidation of natural terpenyl alcohols by gold nanoparticles under O2 followed by original condensation with resorcinol derivatives catalyzed by TiIV supported on montmorillonite with unusual regioselectivity to access ortho-tetrahydrocannabinol structures.
|
Development of Photoactivatable Nitroxyl (HNO) Donors Incorp...
2018-04-15 [10.1002/ejoc.201800092] |
|
Catalytic C‐Alkylation of Pyrroles with Primary Alcohols: Ha...
2018-03-30 [10.1002/ejoc.201800146] |
|
Fluorine‐Containing Functionalized Cyclopentene Scaffolds Th...
2018-03-30 [10.1002/ejoc.201800057] |
|
Verdazyl Radical Building Blocks: Synthesis, Structure, and ...
2018-03-30 [10.1002/ejoc.201701783] |
|
Iron‐Catalyzed Sulfur‐Promoted Decyanative Redox Condensatio...
2018-03-30 [10.1002/ejoc.201701607] |