First hyperpolarizability of isomers of pyridinium N-phenoxide betaine dye in solution using the ASEC-FEG method
E.M. Torres, H.C. Georg, T.L. Fonseca, M.A. Castro
Index: 10.1016/j.cplett.2018.03.070
Full Text: HTML
Abstract
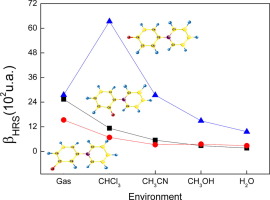
The linear and nonlinear properties of isomeric forms of pyridinium-N-phenoxide betaine dye were investigated in protic and aprotic solvents using atomistic simulations. We employed the sequential Quantum Mechanics/Molecular Mechanics (S-QM/MM) and the free energy gradient (FEG) methods to optimize the geometry of each isomer in chloroform, acetonitrile, methanol and water. The results show a complex dependence of the first hyperpolarizability with respect to the solvent nature and isomeric form, with a marked effect of conformational changes for para-betaine. Large contrasts of the first hyperpolarizability show a clear distinction between isomeric forms in solution that could be experimentally detected.
|
Performance of Kobryn-Gusarov-Kovalenko Closure from a Therm...
2018-04-09 [10.1016/j.cplett.2018.04.013] |
|
Configurational Coupled Cluster Approach with Applications t...
2018-04-08 [10.1016/j.cplett.2018.04.017] |
|
Urea-assisted liquid-phase exfoliation of natural graphite i...
2018-04-07 [10.1016/j.cplett.2018.04.019] |
|
Morphological Evolution of Solution-Grown Cobalt-Doped ZnO N...
2018-04-03 [10.1016/j.cplett.2018.04.002] |
|
Adsorption of Cyanogen Chloride on the surface of Boron Nitr...
2018-04-03 [10.1016/j.cplett.2018.04.001] |