Morphological Evolution of Solution-Grown Cobalt-Doped ZnO Nanostructures and Their Properties
Qui Thanh Hoai Ta, Gitae Namgung, Jin-Seo Noh
Index: 10.1016/j.cplett.2018.04.002
Full Text: HTML
Abstract
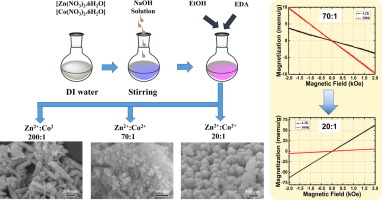
It is demonstrated that the morphology of Co-doped ZnO nanostructures can be easily altered by controlling Co-doping concentration. A facile low-temperature solution method was employed for the nanosynthesis. The morphology of the nanostructures changed from nanorods to nanoparticles, and to needle-like structures as the molar ratio of Co2+ ions increased. No noticeable changes in structural and optical properties were caused by the low concentration of Co-doping, while a magnetic transition was observed. At the very low Co concentrations below 0.3 at%, the nanostructures showed diamagnetism, whereas a paramagnetic behavior was observed at a concentration of 2.5 at%.
|
Performance of Kobryn-Gusarov-Kovalenko Closure from a Therm...
2018-04-09 [10.1016/j.cplett.2018.04.013] |
|
Configurational Coupled Cluster Approach with Applications t...
2018-04-08 [10.1016/j.cplett.2018.04.017] |
|
Urea-assisted liquid-phase exfoliation of natural graphite i...
2018-04-07 [10.1016/j.cplett.2018.04.019] |
|
Adsorption of Cyanogen Chloride on the surface of Boron Nitr...
2018-04-03 [10.1016/j.cplett.2018.04.001] |
|
Broadband Two-Photon Absorption Cross Sections of Benzothiaz...
2018-03-31 [10.1016/j.cplett.2018.03.075] |