Theoretical Studies on the Mechanism and Kinetics of the Reaction of CF3 radical with Oxygen Molecule
Vahid Saheb, Mahdiyeh Javanmardi
Index: 10.1016/j.jfluchem.2018.03.017
Full Text: HTML
Abstract
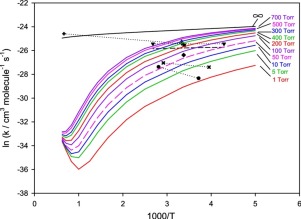
The potential energy surface of the CF3 + O2 reaction is explored by using quantum-chemical combination methods including CBS-QB3, G3B3 and G4. A TST/RRKM model along with a modified strong collision approximation is employed to calculate the thermal rate coefficients for important product channels as a function of temperature and pressure. The calculated results show that the overall rate constant is pressure-dependent over a wide temperature range. At low temperatures, the dominant process is the formation of CF3OO adduct with negative activation energy. However, at higher temperatures, the product channel CF3O + O becomes important. The unimolecular rate coefficients for the thermal decomposition of CF3OO are also computed.
|
Synthesis and Characterization of Pentafluorosulfanyl-Functi...
2018-04-07 [10.1016/j.jfluchem.2018.04.004] |
|
Characterization of electronic features of intermolecular in...
2018-03-30 [10.1016/j.jfluchem.2018.03.016] |
|
Structure and photoluminescence properties study of neodymiu...
2018-03-30 [10.1016/j.jfluchem.2018.03.015] |
|
Study of Fluoride Content in Some Commercial Phosphate Ferti...
2018-03-29 [10.1016/j.jfluchem.2018.03.018] |
|
Straightforward synthesis of fluorinated amino acids by Mich...
2018-03-29 [10.1016/j.jfluchem.2018.03.014] |