|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |

![5-[[4-[(3,4,5-trimethoxyphenyl)methyl]oxolan-3-yl]methyl]benzo[1,3]dioxole Structure](https://image.chemsrc.com/caspic/276/73465-36-8.png)
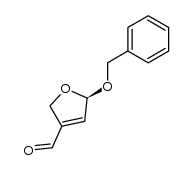
![benzo[d][1,3]dioxol-5-yl((S)-5-(benzyloxy)-2,5-dihydrofuran-3-yl)methanol Structure](https://image.chemsrc.com/caspic/360/120040-58-6.png)
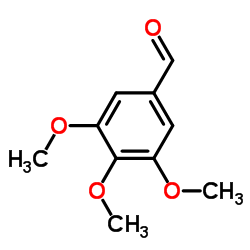

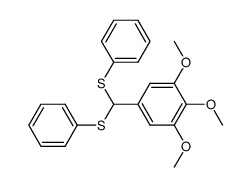
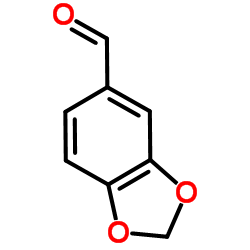
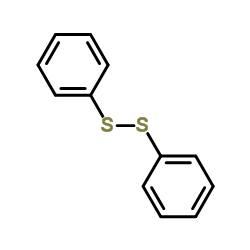
![1,3-Benzodioxole,5-[bis(phenylthio)methyl] Structure](https://image.chemsrc.com/caspic/447/6302-93-8.png)
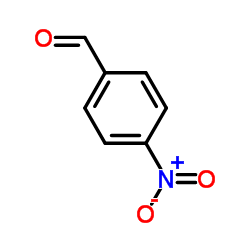
![Benzene,1-[bis(phenylthio)methyl]-4-nitro Structure](https://image.chemsrc.com/caspic/017/23837-16-3.png)