|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

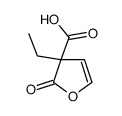
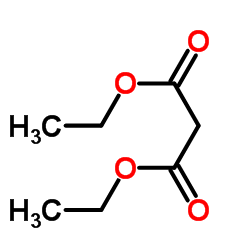

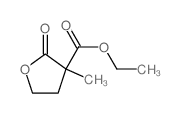
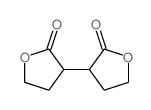
![2,2'-dioxo-tetrahydro-[3,3']bifuryl-3,3'-dicarboxylic acid diethyl ester Structure](https://image.chemsrc.com/caspic/198/859931-22-9.png)

![3-oxo-2-oxa-bicyclo[2.1.1]hexane-4-carboxylic acid ethyl ester Structure](https://image.chemsrc.com/caspic/267/859175-75-0.png)
![3,7-dioxabicyclo[3.2.1]octane-4,6-dione Structure](https://image.chemsrc.com/caspic/330/7473-08-7.png)