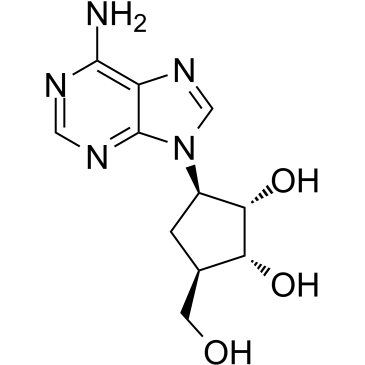
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
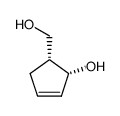
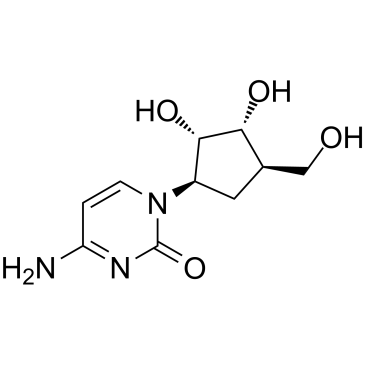
![(3S,3aS,6aR)-2-oxo-3,3a,4,6a-tetrahydro-2H-cyclopenta[b]furan-3-yl (S)-2-acetoxypropanoate Structure](https://image.chemsrc.com/caspic/480/160154-62-1.png)
![acetic acid,[(1R,5R)-5-(hydroxymethyl)cyclopent-2-en-1-yl] acetate Structure](https://image.chemsrc.com/caspic/221/178456-34-3.png)