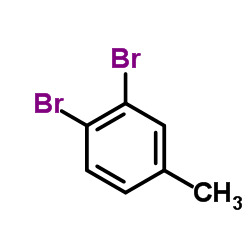
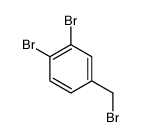
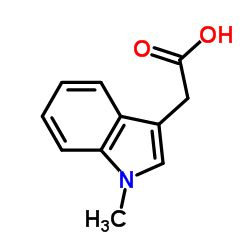
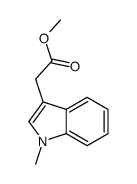
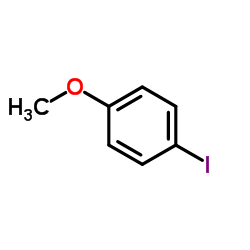
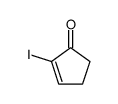
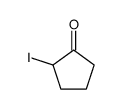
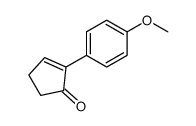
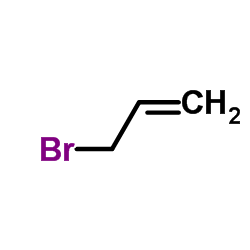
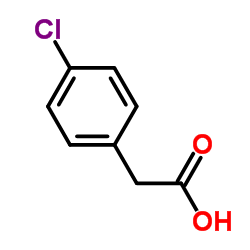
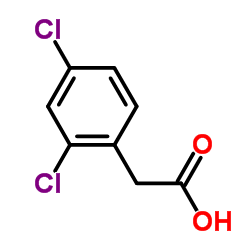
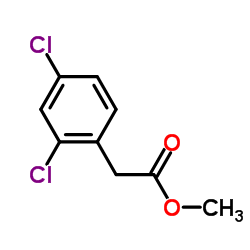
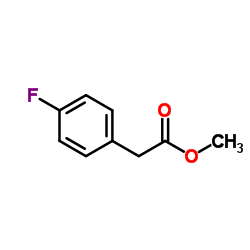
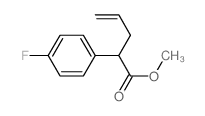
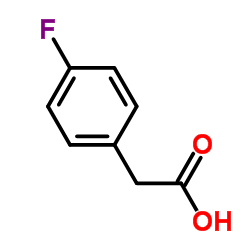
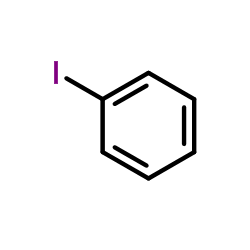
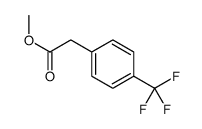
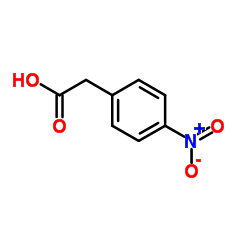
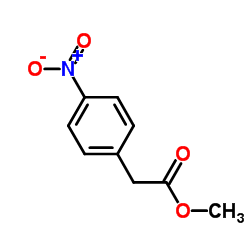
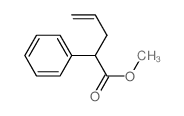
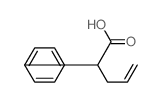
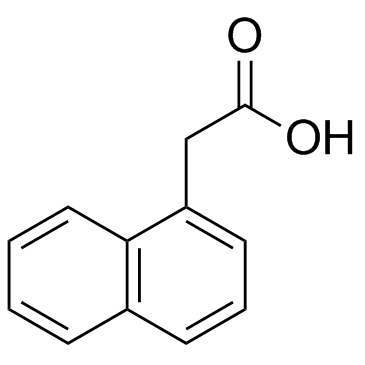
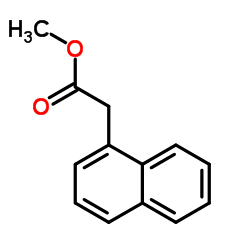
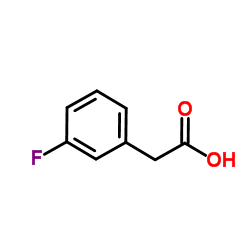
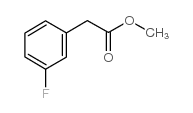
|
~90% |
|
~99% |
|
~% |
|
~75% |
|
~% |
|
~99% |
|
~95% |
|
~% |
|
~24% |
|
~99% |
|
~99% |
|
~99% |
|
~99% |
|
~98% |
|
~99% |
|
~16% |
|
~% |