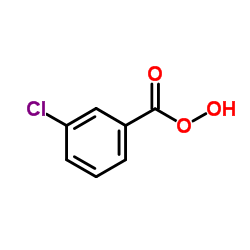
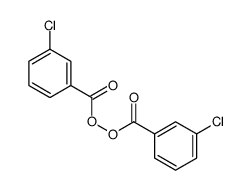
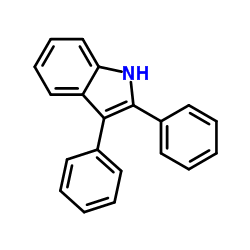
|
~95% |
|
~40% |
|
~0% |
|
~95% |
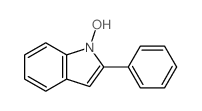
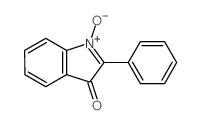
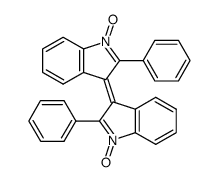
![1'-hydroxy-2,2'-diphenyl-1,2-dihydro-1'H-[2,3']biindolyl-3-one Structure](https://image.chemsrc.com/caspic/133/58535-63-0.png)