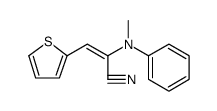
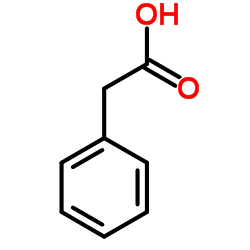
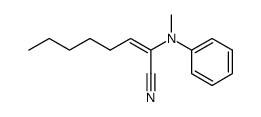
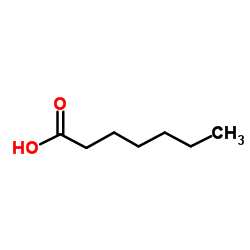
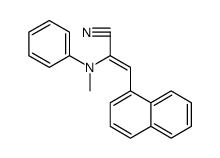
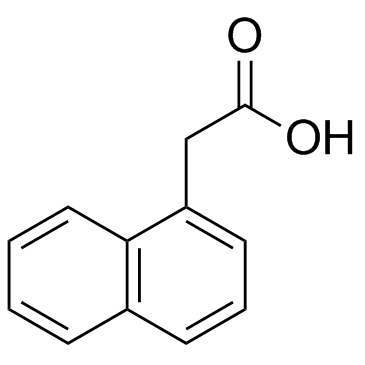
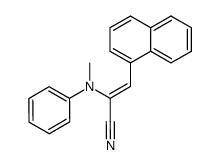
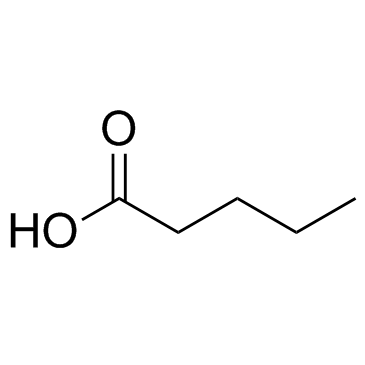
|
~83% |
|
~87% |
|
~78% |
|
~75% |
|
~98% |
|
~96% |
|
~76% |