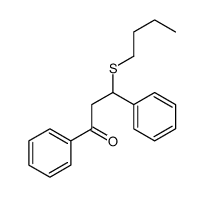
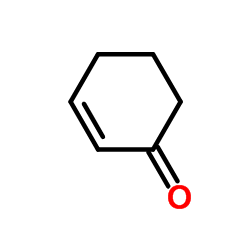
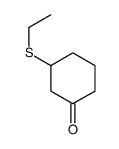
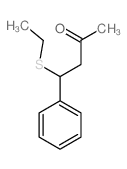
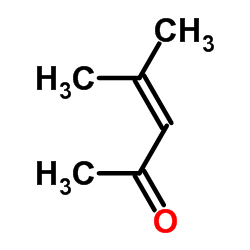
|
~93% |
|
~% |
|
~95% |
|
~92% |
|
~92% |
|
~% |
|
~96% |
|
~94% |
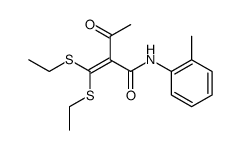
![2-[bis(benzylthio)methylene]-3-oxo-N-o-tolylbutanamide Structure](https://image.chemsrc.com/caspic/380/875778-80-6.png)
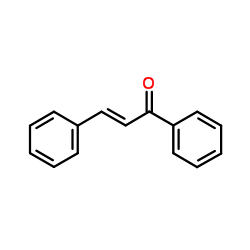
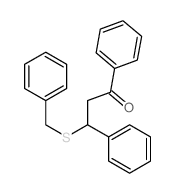
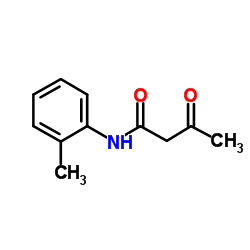
![2-[bis(butylthio)methylene]-3-oxo-N-o-tolylbutanamide Structure](https://image.chemsrc.com/caspic/339/885517-59-9.png)