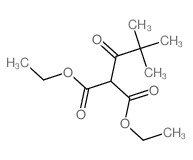
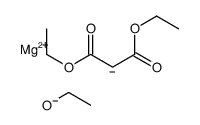
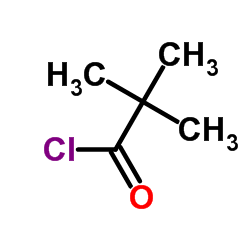
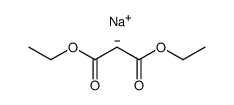
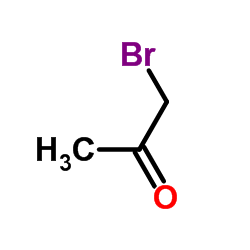
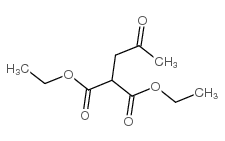
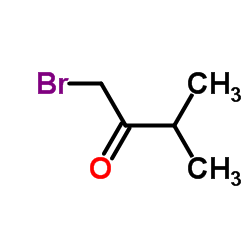
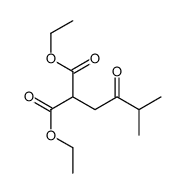
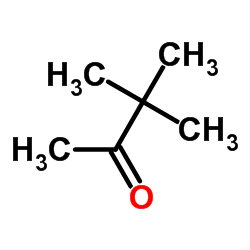
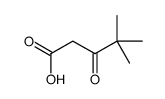
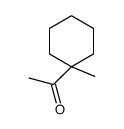
|
~30% |
|
~74% |
|
~% |
|
~75% |
|
~78% |
|
~38% |
|
~49% |
![1-[Bis(ethoxycarbonyl)methyl]-2,2-dimethylcyclopropanol Structure](https://image.chemsrc.com/caspic/251/77320-47-9.png)