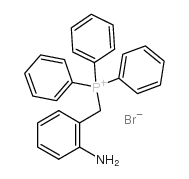
|
~99% |
|
~% |
|
~% |
|
~% |
|
~83% |
|
~% |

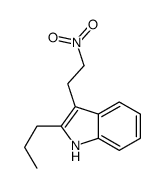

![[2-(Butanamido)benzyl]triphenylphosphonium bromide Structure](https://image.chemsrc.com/caspic/261/140858-52-2.png)