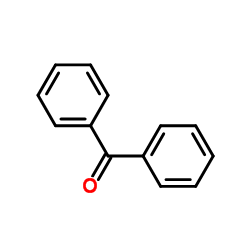
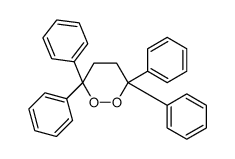
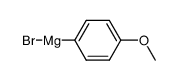
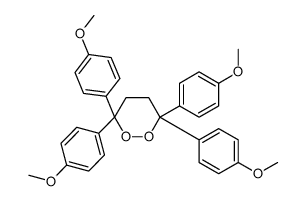
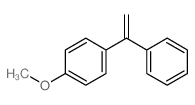
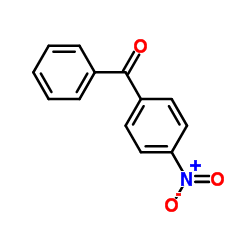
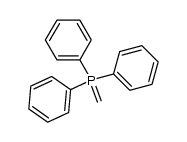
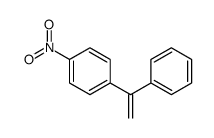
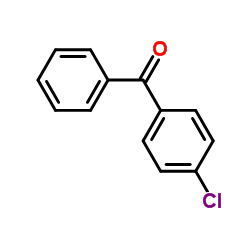
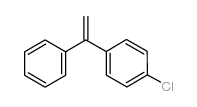
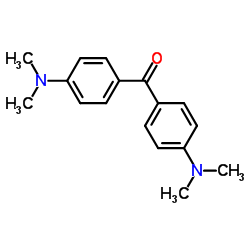
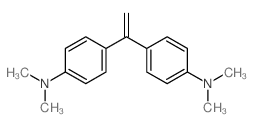
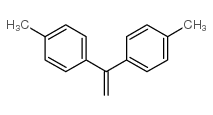
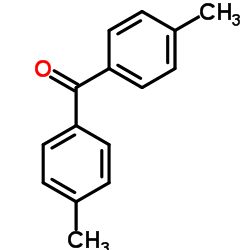
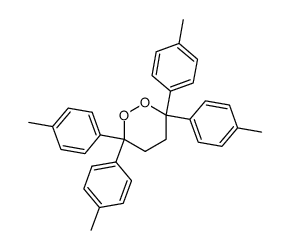
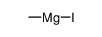|
~% |
|
~17%
Detail
|
|
~% |
|
~63% |
|
~20% |
|
~71% |
|
~40% |
|
~67% |
|
~46% |
|
~0% |
|
~79% |
![1-nitro-4-[1-(4-nitrophenyl)ethenyl]benzene Structure](https://image.chemsrc.com/caspic/272/10605-46-6.png)