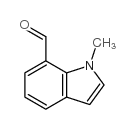
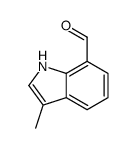
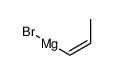
|
~29% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~99% |
|
~89% |
|
~68% |
|
~17% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
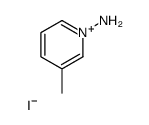

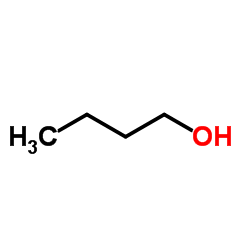

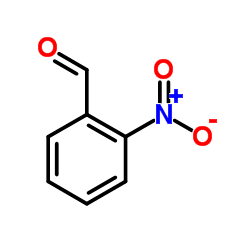
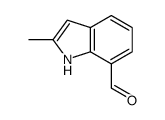

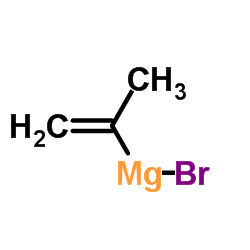
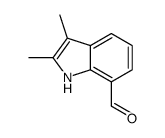

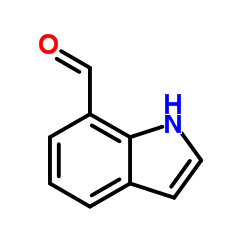
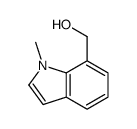
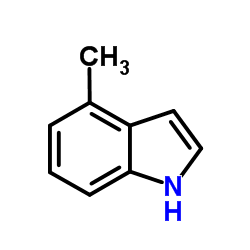

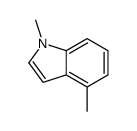
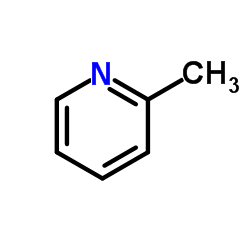
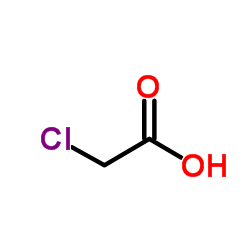
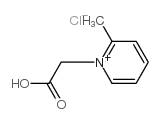
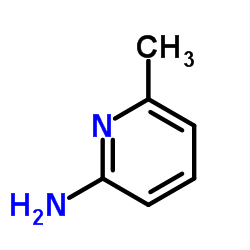
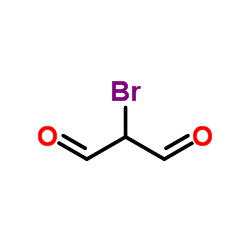
![5-methylimidazo[1,2-a]pyridine-3-carbaldehyde Structure](https://image.chemsrc.com/caspic/450/178488-37-4.png)
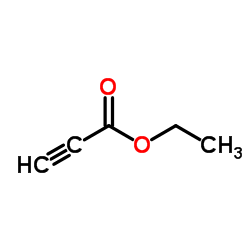
![ethyl 4-methylpyrazolo[1,5-a]pyridine-3-carboxylate Structure](https://image.chemsrc.com/caspic/429/55899-17-7.png)
![ethyl 6-methylpyrazolo[1,5-a]pyridine-3-carboxylate Structure](https://image.chemsrc.com/caspic/391/55899-18-8.png)