|
~78% |
|
~% |
|
~99% |
|
~% |
|
~80% |
|
~% |
|
~% |

![Benzoicacid,4-[(1R)-1-aminoethyl]-,methylester Structure](https://image.chemsrc.com/caspic/404/912342-10-0.png)
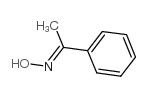
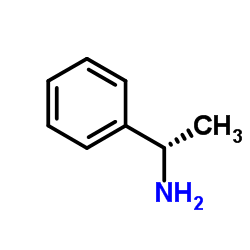
![2,2,2-Trifluoro-N-[(1S)-1-phenylethyl]acetamide Structure](https://image.chemsrc.com/caspic/196/2354-91-8.png)

