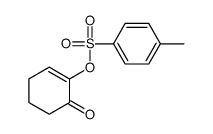
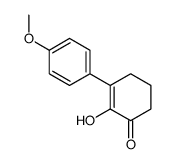
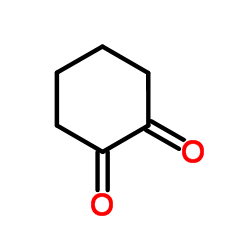
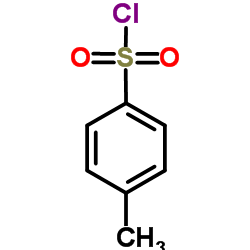
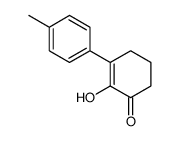
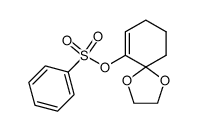
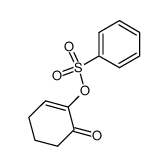
|
~50% |
|
~% |
|
~60% |
|
~44% |
|
~% |
|
~36% |
|
~39% |
|
~% |
|
~63% |