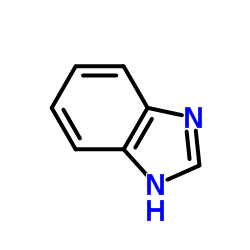
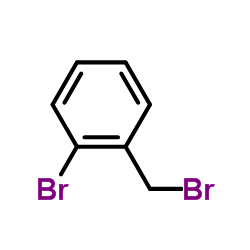
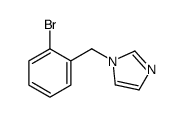
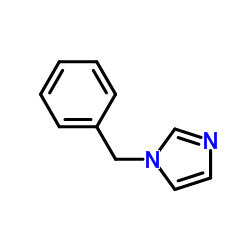
|
~64% |
|
~51% |
|
~76% |
|
~57% |
![1-[(2-bromophenyl)methyl]benzimidazole Structure](https://image.chemsrc.com/caspic/363/312631-76-8.png)

![4-[(2-bromobenzyl)oxy]-6-methyl-2H-pyran-2-one Structure](https://image.chemsrc.com/caspic/172/1033480-91-9.png)
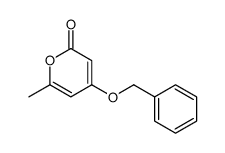
![3-methyl-1H,6H-pyrano[4,3-c]isochromen-1-one Structure](https://image.chemsrc.com/caspic/492/1033481-33-2.png)