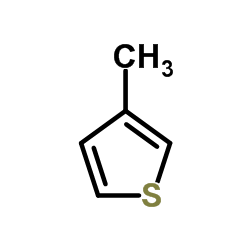
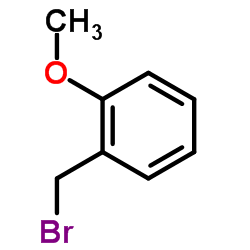
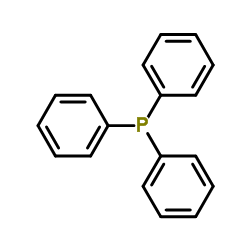
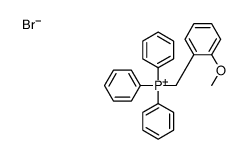
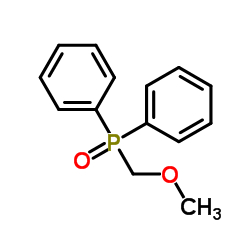
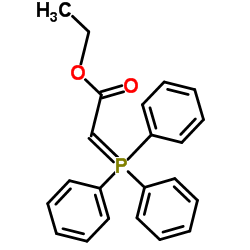
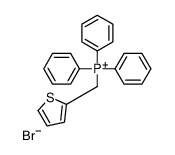
|
~99% |
|
~43% |
|
~85% |
|
~54% |
|
~99% |
|
~57% |
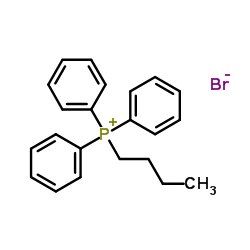
![[butyl(phenyl)phosphoryl]benzene Structure](https://image.chemsrc.com/caspic/100/4233-13-0.png)