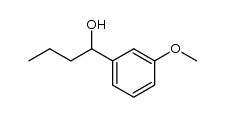
|
~69% |
|
~92% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~75% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |

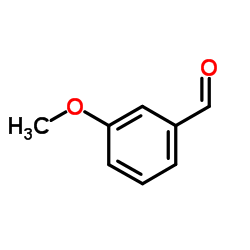
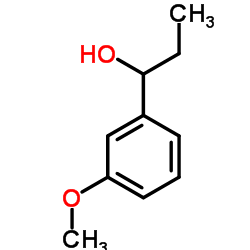
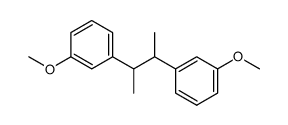
![3-[3-(3-hydroxyphenyl)butan-2-yl]phenol Structure](https://image.chemsrc.com/caspic/162/68266-23-9.png)
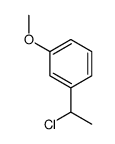


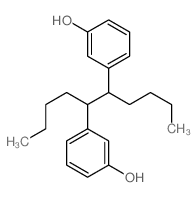

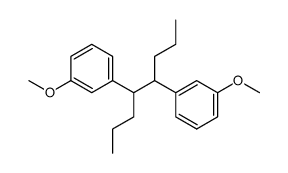
![3-[5-(3-hydroxyphenyl)octan-4-yl]phenol Structure](https://image.chemsrc.com/caspic/076/68266-25-1.png)