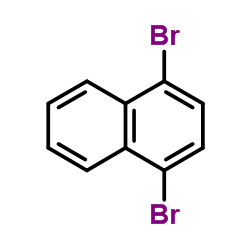
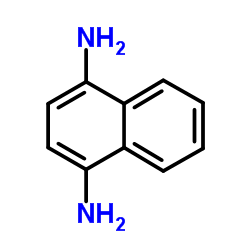
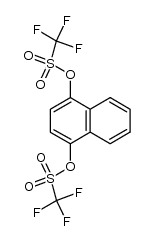
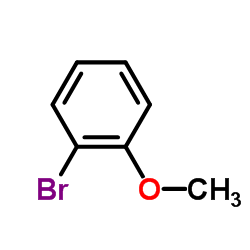
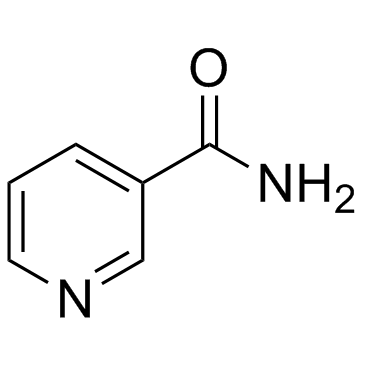
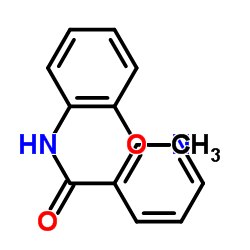
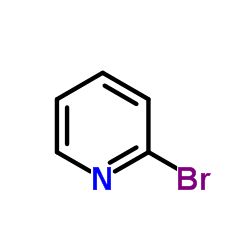
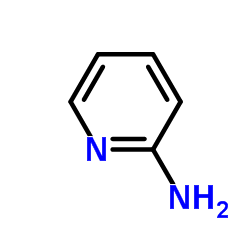
|
~66% |
|
~57% |
|
~53% |
|
~36% |
|
~% |
![6-Bromo-2,3-dihydrobenzo[b][1,4]dioxine Structure](https://image.chemsrc.com/caspic/013/52287-51-1.png)