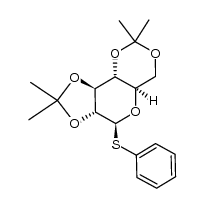
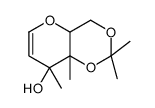
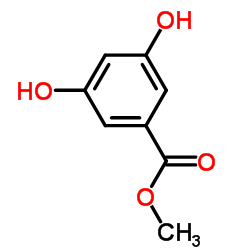
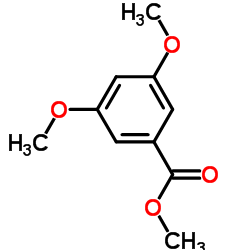
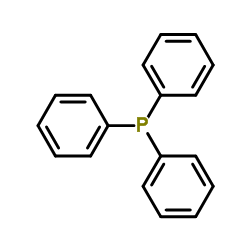
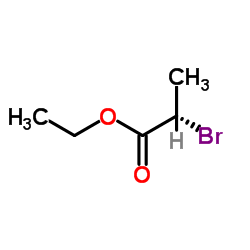
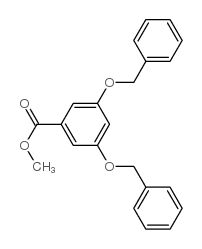
|
~99% |
|
~99% |
|
~98% |
|
~89% |
|
~10% |