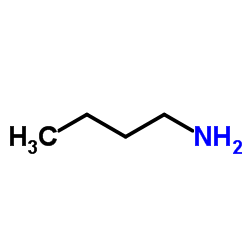
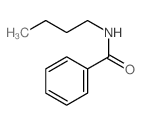
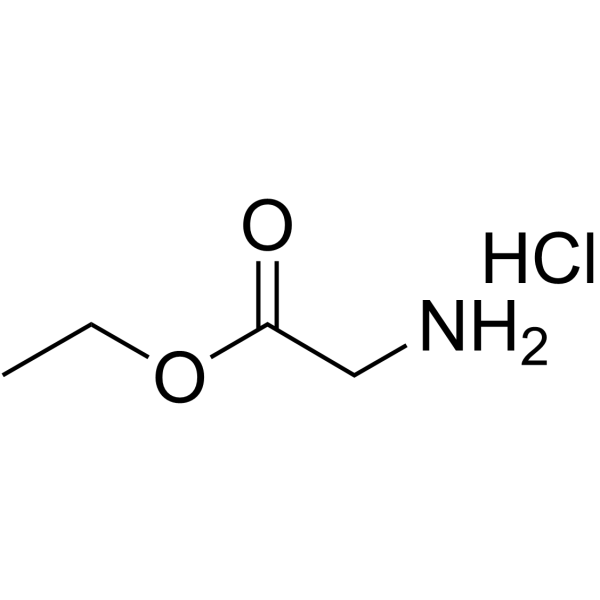
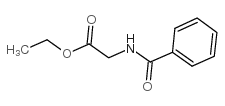
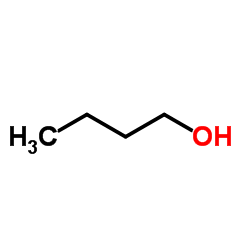
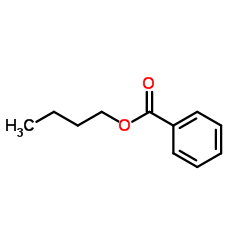
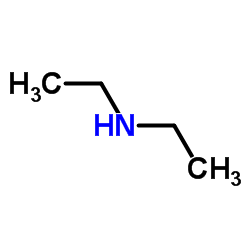
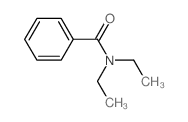
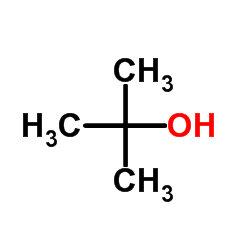
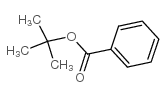
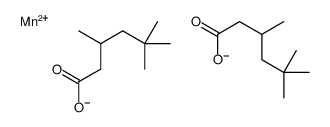
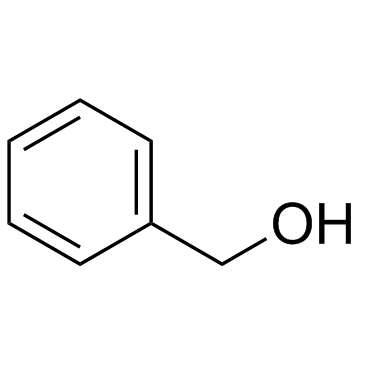
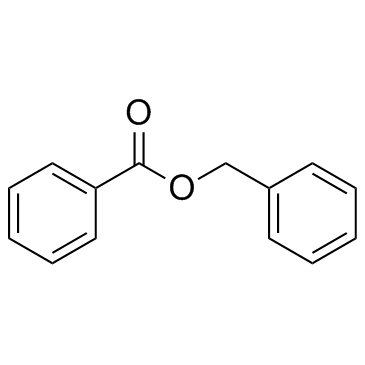
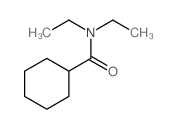
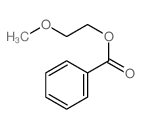
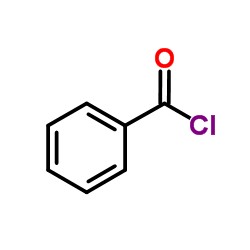
|
~82% |
|
~39% |
|
~79% |
|
~83% |
|
~73% |
|
~10% |
|
~65% |
|
~% |
|
~75% |
|
~58% |
|
~42% |
|
~49%
Detail
|
|
~71% |
|
~% |