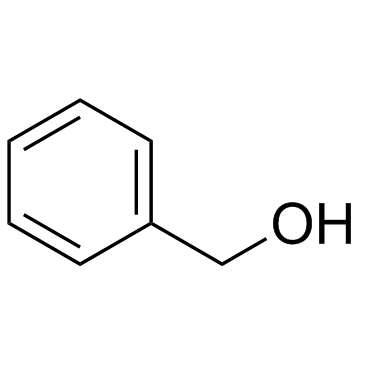
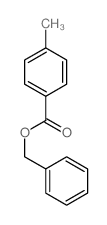
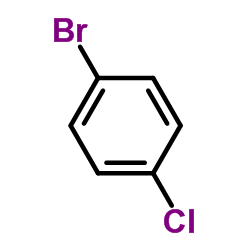
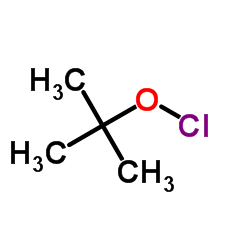
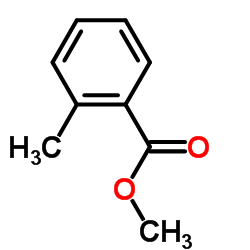
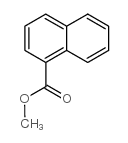
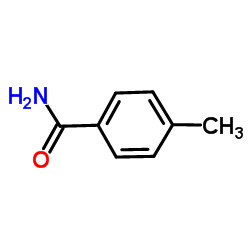
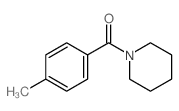
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~82% |
|
~91% |
|
~90% |
|
~84% |
|
~65% |
|
~% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~%
Detail
|
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
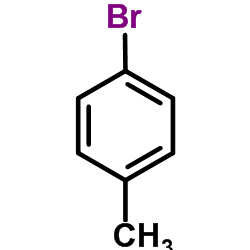
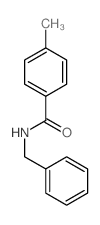
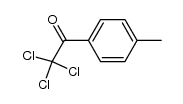
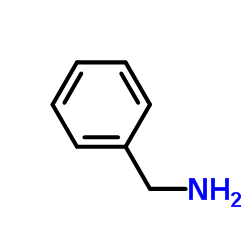


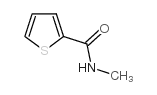
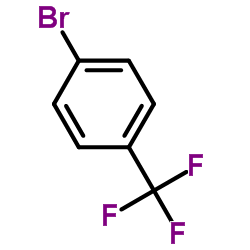
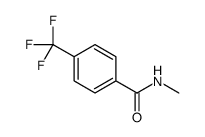
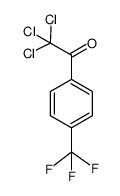

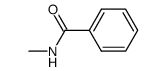
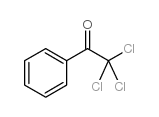
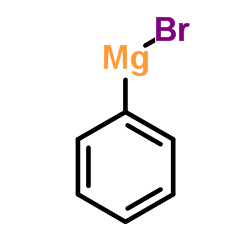
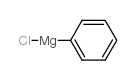
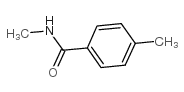

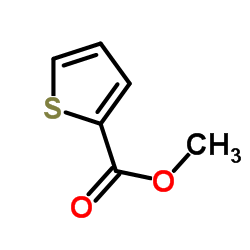
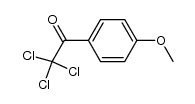
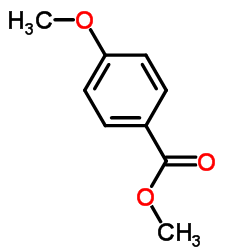
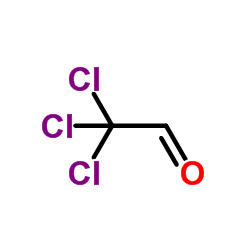
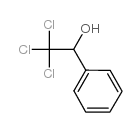
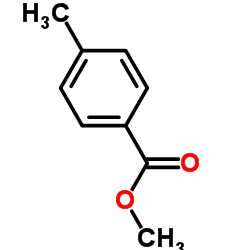
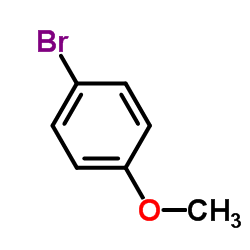

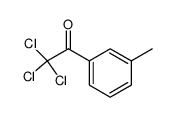
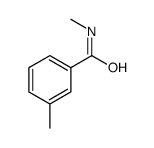
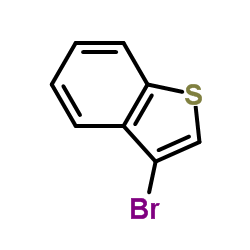
![Benzo[b]thiophene-3-carboxylic acid methyl ester Structure](https://image.chemsrc.com/caspic/179/22913-25-3.png)