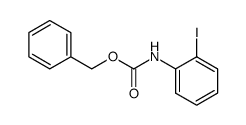
|
~89% |
|
~17% |
|
~% |
|
~% |
|
~% |
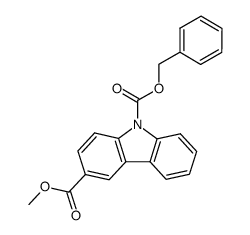
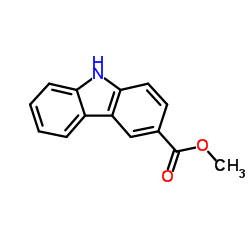
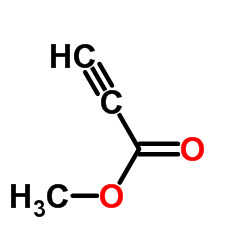
![N-(carbobenzyloxy)-2-[(E)-2-(phenylthio)ethenyl]indole Structure](https://image.chemsrc.com/caspic/466/522614-60-4.png)