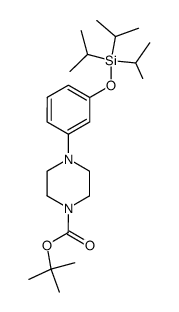
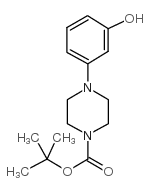
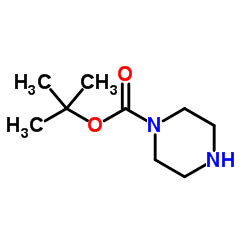
|
~% |
|
~% |
|
~% |
|
~% |
|
~81% |
|
~99% |
|
~% |
|
~% |

![8-(3-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PHENYL)-1,4-DIOXA-8-AZASPIRO[4.5]DECANE Structure](https://image.chemsrc.com/caspic/214/862261-25-4.png)
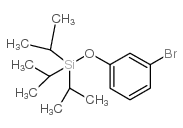
![8-(3-((triisopropylsilyl)oxy)phenyl)-1,4-dioxa-8-azaspiro[4.5]decane Structure](https://image.chemsrc.com/caspic/252/862261-24-3.png)
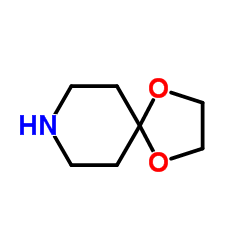
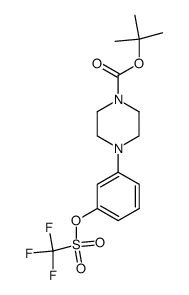
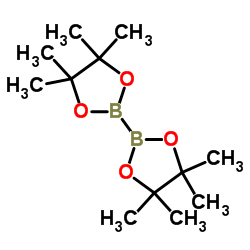
![3-[4-(N-Boc)piperazin-1-yl]phenylboronic acid pinacol ester Structure](https://image.chemsrc.com/caspic/354/540752-87-2.png)