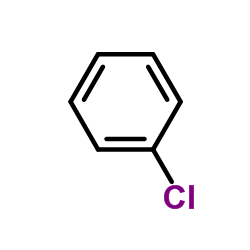
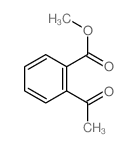
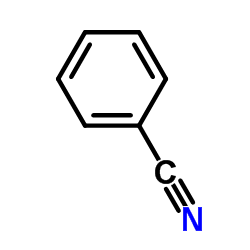
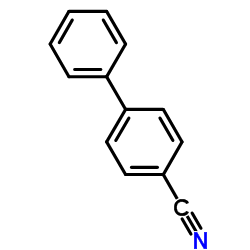
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |


![1-([1,1'-Biphenyl]-2-yl)ethanone Structure](https://image.chemsrc.com/caspic/270/2142-66-7.png)