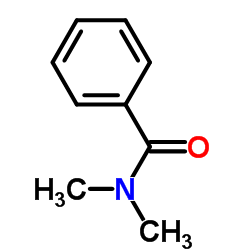
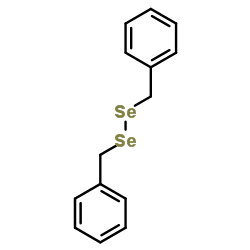
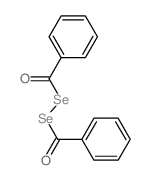
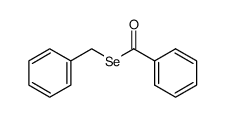
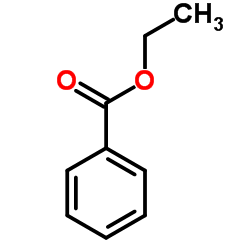
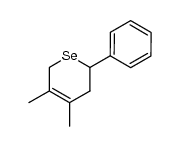
|
~0% |
|
~5% |
|
~0% |
|
~0% |
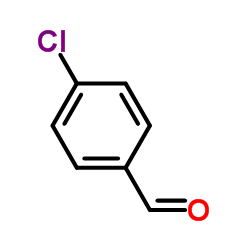

![Diselenide,bis[(4-chlorophenyl)methyl] (9CI) Structure](https://image.chemsrc.com/caspic/253/56344-11-7.png)