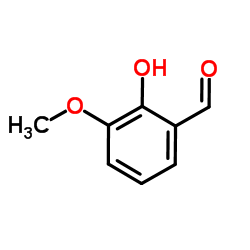
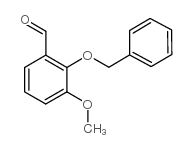
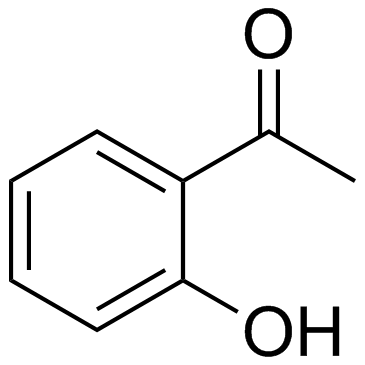
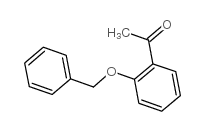
|
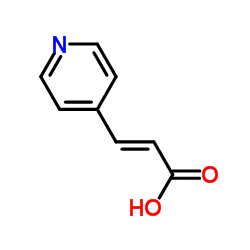
~95% |
|
~%
Detail
|
|
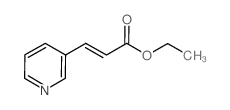
~97% |
|
~% |
|
~% |
|
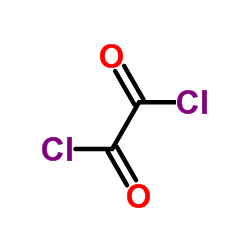
~93% |
|
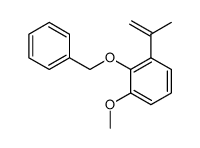
~91% |