|
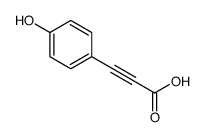
~72% |
|
~% |
|
~% |
|
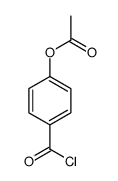
~56% |
|
~% |
|
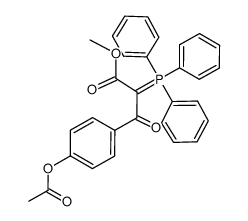
~39% |
|
~% |
|
~% |
|
~% |
|
~% |
|
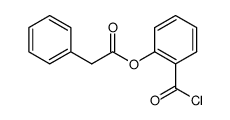
~71% |

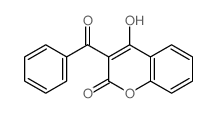
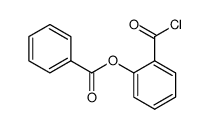
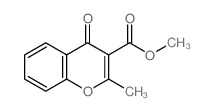

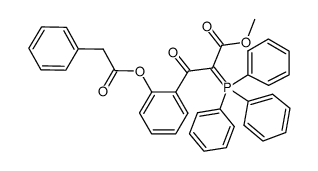
![11-hydroxybenzo[b]xanthen-12-one Structure](https://image.chemsrc.com/caspic/471/5530-11-0.png)