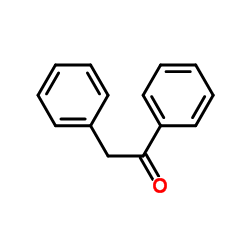
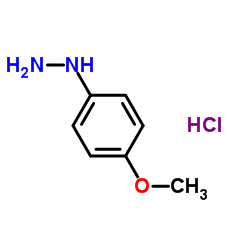
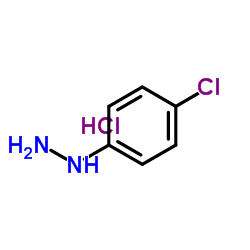
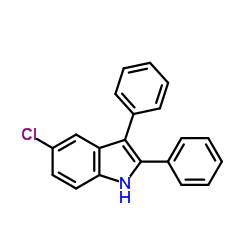
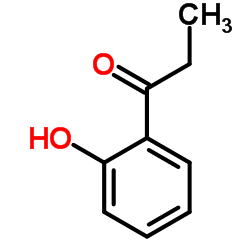
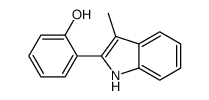
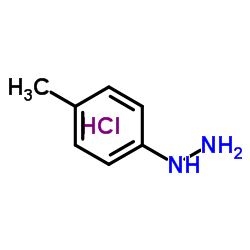
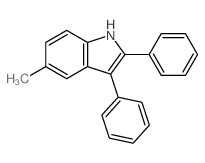
|
~41% |
|
~81% |
|
~94% |
|
~89% |