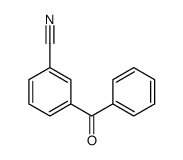
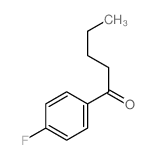
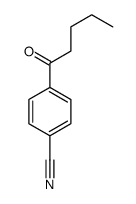
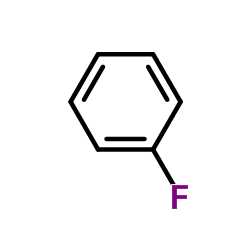
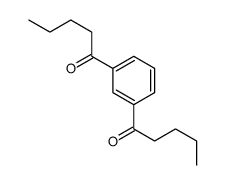
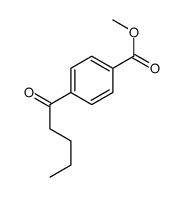
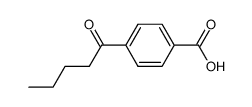
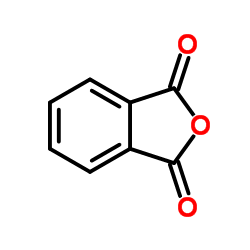
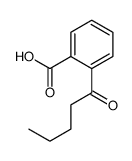
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~44% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
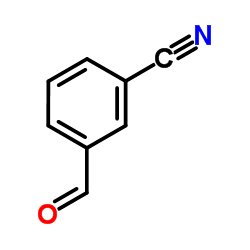
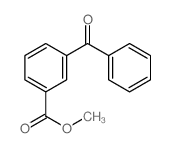
![3-[hydroxy(phenyl)methyl]benzonitrile Structure](https://image.chemsrc.com/caspic/453/13428-06-3.png)