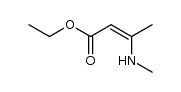
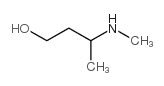
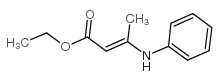
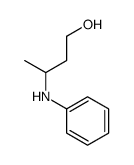
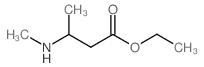
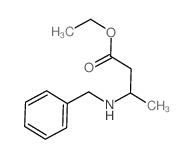
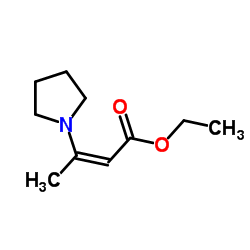
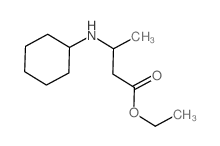
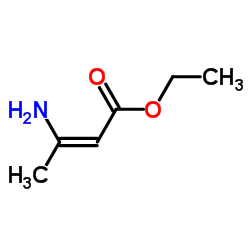
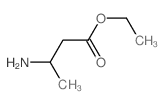
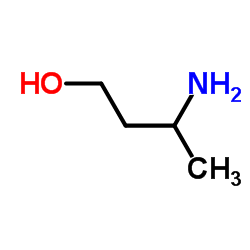
|
~64% |
|
~56% |
|
~71% |
|
~88% |
|
~79% |
|
~85% |
|
~71% |
|
~63% |