|
~% |
|
~% |
|
~% |
|
~77% |
|
~% |
|
~74% |
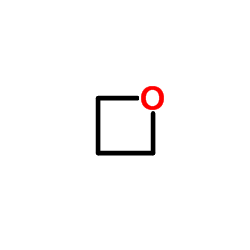
![Spiro[cyclohexane-1,2'-oxetane] Structure](https://image.chemsrc.com/caspic/047/185-18-2.png)

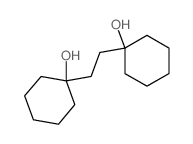
![1-Oxaspiro[4.5]decane Structure](https://image.chemsrc.com/caspic/116/176-91-0.png)
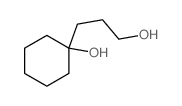
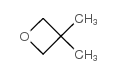


![1-oxaspiro[4.5]dec-6-ene Structure](https://image.chemsrc.com/caspic/407/7129-25-1.png)