CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DB1290000
-
CHEMICAL NAME :
-
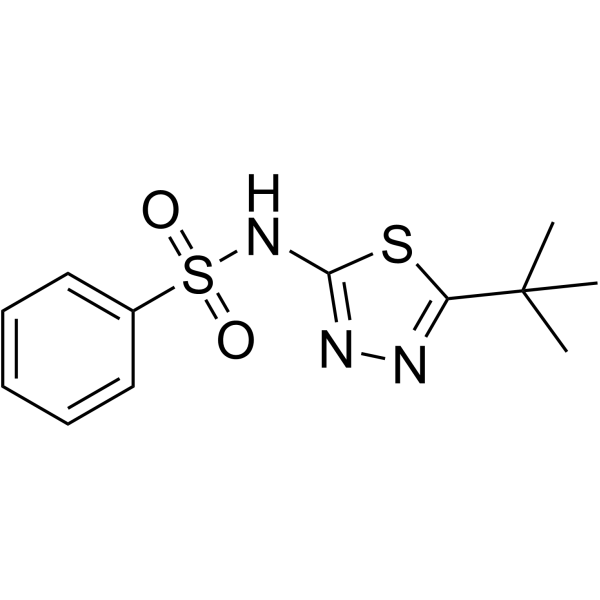
Benzenesulfonamide, N-(5-tert-butyl-1,3,4-thiadiazol-2-yl)-
-
CAS REGISTRY NUMBER :
-
1492-02-0
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
13
-
MOLECULAR FORMULA :
-
C12-H15-N3-O2-S2
-
MOLECULAR WEIGHT :
-
297.42
-
WISWESSER LINE NOTATION :
-
T5NN DSJ CX1&1&1 EMSWR
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
219 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
310 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
550 mg/kg
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - dyspnea Endocrine - hypoglycemia
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,131,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
235 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
248 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
193 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
967 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
118 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2100 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2100 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1050 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YIKUAO Yamaguchi Igaku. Yamaguchi Medicine. (Yamaguchi Daigaku Igakkai, Kogushi Ube, Yamaguchi- Ken, Japan) V.1- 1953- Volume(issue)/page/year: 18,21,1969
|