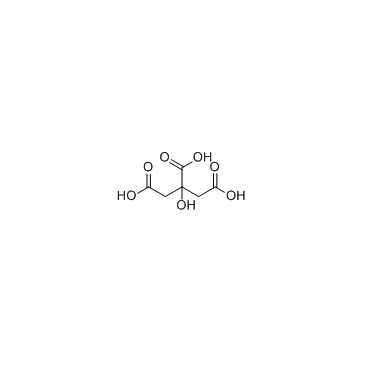
2338-05-8
| 中文名 | 柠檬酸铁四水合物 |
|---|---|
| 英文名 | ferric citrate |
| 中文别名 |
2,3-二氟-6-甲氧基苯甲醛
柠檬酸铁 |
| 英文别名 |
iron citrate
MFCD00013098 EINECS 219-045-4 2,3-DIFLUORO-6-METHOXY-BENZALDEHYD |
| 沸点 | 309.6ºC at 760mmHg |
|---|---|
| 分子式 | C6H5FeO7 |
| 分子量 | 244.94500 |
| 闪点 | 155.2ºC |
| 精确质量 | 244.93800 |
| PSA | 99.13000 |
| 外观性状 | 红色至棕色粉末 |
| 蒸汽压 | 5.73E-05mmHg at 25°C |
| 储存条件 | 室温,干燥 |
| 稳定性 | Stable. Light sensitive. |
| 分子结构 | 1、摩尔折射率:无可用的 2、摩尔体积(cm3/mol):无可用的 3、等张比容(90.2K):无可用的 4、表面张力(dyne/cm):无可用的 5、介电常数:无可用的 6、极化率(10-24cm3):无可用的 7、单一同位素质量:244.93847Da 8、标称质量:245Da 9、平均质量:244.9447Da |
| 计算化学 | 1、 疏水参数计算参考值(XlogP): 2、 氢键供体数量:5 3、 氢键受体数量:11 4、 可旋转化学键数量:2 5、 互变异构体数量: 6、 拓扑分子极性表面积(TPSA):145 7、 重原子数量:18 8、 表面电荷:0 9、 复杂度:211 10、同位素原子数量:0 11、确定原子立构中心数量:0 12、不确定原子立构中心数量:0 13、确定化学键立构中心数量:0 14、不确定化学键立构中心数量:0 15、共价键单元数量:6 |
| 更多 | 1. 性状:红褐色透明小薄片或褐色粉末 2. 密度(g/L,20ºC):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):未确定 5. 沸点(ºC,常压):未确定 6. 沸点(ºC 0.4mmHg):未确定 7. 折射率(nD20):未确定 8. 闪点(ºF):未确定 9. 比旋光度():未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa,25ºC):未确定 12. 饱和蒸气压(kPa,20ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:在水中溶解缓慢,热水比冷水易溶。不溶于乙醇。 |
|
Section 1. Chemical Product and Company Identification Catalog Ferric Citrate Common Name/YY1591, F1028 Number(s). Trade Name CAS#2338-05-8(anhydrous CAS
no.) Manufacturer Commercial Name(s) Ferric Citrate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Cold water may be used. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Cold water may be used. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Not available. Flash Points Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2). Some metallic oxides. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various Substances Slightly explosive in presence of open flames and sparks. Explosion Hazards in Non-explosive in presence of shocks. Presence of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media LARGE FIRE: Use water spray, fog or foam. Do not use water jet. and Instructions Special Remarks onAs with most organic solids, fire is possible at elevated temperatures Fire Hazards Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust explosion hazard. Hazards Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Section 7. Handling and Storage Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do Precautions not ingest. Do not breathe dust. Wear suitable protective clothing. If ingested, seek medical advice immediately and show the container or the label. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Sensitive to light. Store in light-resistant containers. Ferric Citrate Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be a Large Spillused to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. TWA: 1 (mg(Fe)/m3) from ACGIH (TLV) [United States] Exposure Limits TWA: 1 (mg(Fe)/m3) from NIOSH [United States] TWA: 1 STEL: 2 (mg(Fe)/m3) [Canada] TWA: 1 STEL: 2 (mg((Fe)/m3) [United Kingdom (UK)] Consult local authorities for acceptable exposure limits. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Powdered solid.)OdorNot available. TasteNot available. Molecular Weight244.95 g/mole + xH20 pale-brown or grayish-brown Color Not available. pH (1% soln/water) Boiling PointNot available. Not available. Melting Point Critical TemperatureNot available. Specific GravityNot available. Vapor PressureNot applicable. Vapor DensityNot available. Not available. Volatility Odor ThresholdNot available. Not available. Water/Oil Dist. Coeff. Ionicity (in Water)Not available. Dispersion PropertiesSee solubility in water. SolubilityEasily soluble in hot water. Soluble in cold water. Slowly, but completely soluble in cold water. Readily soluble in hot water. Insoluble in alcohol. Section 10. Stability and Reactivity Data StabilityThe product is stable. Instability TemperatureNot available. Conditions of InstabilityExcess heat, incompatible materials, dust generation, light Incompatibility with various Reactive with oxidizing agents. substances Not available. Corrosivity Special Remarks onSensitive to light. Reactivity Ferric Citrate Not available. Special Remarks on Corrosivity PolymerizationWill not occur. Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Acute oral toxicity (LD50): 1487 mg/kg [Rat]. Toxicity to Animals Acute dermal toxicity (LD50): 2000 mg/kg [Rabbit]. Chronic Effects on Humans May cause damage to the following organs: liver. Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Other Toxic Effects on Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: May cause eye irritation. Inhalation: This material may produce dust. May cause upper respiratory tract (nose, throat) irritation. Symptoms may include coughing, sore throat, labored breathing and chest pain. Breathing small amounts of this material during normal handling is not likely to cause harmful effects. Ingestion: Swallowing small amounts during normal handling is not likely to cause harmful effects. Swallowing large amounts of iron may be harmful and may cause gastrointestinal upset (nausea, vomiting, diarrhea) and may also affect the liver. Furthermore, pink urine is a strong indicator of iron poisoning. Chronic Potential Health Effects: Ingestion: Prolonged or repeated ingestion of large quantities may cause liver damage. Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. Toxicity of the ProductsThe product itself and its products of degradation are not toxic. of Biodegradation Not available. Special Remarks on the Products of Biodegradation Section 13. Disposal Considerations Waste must be disposed of in accordance with federal, state and local environmental Waste Disposal control regulations. Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). Not applicable. Identification Special Provisions forNot applicable. Transport Ferric Citrate DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms Rhode Island RTK hazardous substances: Listed as Iron soluble salts Federal and State Pennsylvania RTK: Listed as Iron salts Regulations Minnesota: Listed as Iron soluble salts California Director's List of Hazardous Substances: Listed as Iron soluble salts TSCA 8(b) inventory: Ferric Citrate CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has Proposition 65found to cause cancer which would require a warning under the statute: No products were found. Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsOSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200). EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No. 219-045-4). Canada: Listed on Canadian Domestic Substance List (DSL). China: Not listed on National Inventory. Japan: Listed on National Inventory (ENCS). Korea: Not listed on National Inventory (KECI). Philippines: Listed on National Inventory (PICCS). Australia: Listed on AICS. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications DSCL (EEC)This product is not classifiedNot applicable. according to the EU regulations. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Ferric Citrate Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
| WGK德国 | 3 |
|---|---|
| 海关编码 | 2918150000 |
|
~55% 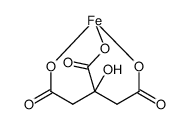
2338-05-8 |
| 文献:Hsiao, Yih-Ming; Chiu, Hsueh-Hung; Wang, Hsiu-Ching; Chang, Yu-Chen Patent: US2005/80283 A1, 2005 ; Location in patent: Page/Page column 2 ; |
| 海关编码 | 2918150000 |
|---|---|
| 中文概述 | 2918150000. 柠檬酸盐和柠檬酸酯. 增值税率:17.0%. 退税率:13.0%. 监管条件:4ABxy(出口许可证,入境货物通关单,出境货物通关单出口许可证(加工贸易),出口许可证(边境小额贸易)). 最惠国关税:6.5%. 普通关税:30.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| 监管条件 | 4.出口许可证 A.入境货物通关单 B.出境货物通关单 x.出口许可证(加工贸易) y.出口许可证(边境小额贸易) |
| 检验检疫 | R.进口食品卫生监督检验 S.出口食品卫生监督检验 N.出口商品检验 |
| Summary | 2918150000. other salts and esters of citric acid. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:4ABXY(export license,certificate of inspection for goods inward,certificate of inspection for goods outwardexport license(processing trade),export license(border small volume trade)). MFN tariff:6.5%. General tariff:30.0% |