Bioscience, Biotechnology, and Biochemistry
1996-09-01
Synthesis of 7-methoxyapigeninidin and its fungicidal activity against Gloeocercospora sorghi.
Y Aida, S Tamogami, O Kodama, T Tsukiboshi
文献索引:Biosci. Biotechnol. Biochem. 60(9) , 1495-6, (1996)
全文:HTML全文
摘要
In the structure of sakuranetin, which was isolated as a phytoalexin from the rice plant, the methoxy group at C-7 has been shown to be important for its high activity. Apigeninidin was isolated as a phytoalexin from sorghum, but it had no methoxy group at C-7. We prepared 7-methoxyapigeninidin and compared its fungicidal activity with that of apigeninidin. The 7-methoxyapigeninidin showed higher activity against sorghum fungi than apigeninidin, suggesting that the methoxy group at C-7 was important for the high fungicidal activity.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
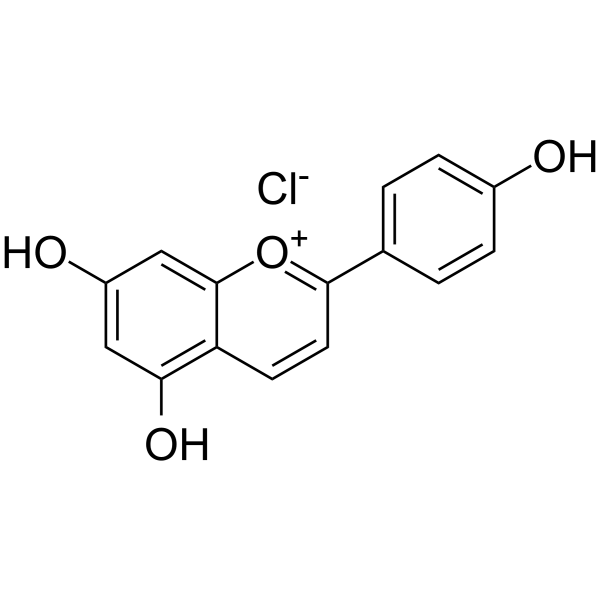 |
氯化芹菜定
CAS:1151-98-0 |
C15H11ClO4 |
相关文献:
更多...
|
Novel pyrano and vinylphenol adducts of deoxyanthocyanidins ...
2014-11-26 [J. Agric. Food Chem. 62(47) , 11536-46, (2014)] |
|
Evaluation of the effect of germination on phenolic compound...
2005-04-06 [J. Agric. Food Chem. 53(7) , 2581-8, (2005)] |
|
Uncommonly high levels of 3-deoxyanthocyanidins and antioxid...
2011-02-23 [J. Agric. Food Chem. 59(4) , 1178-84, (2011)] |
|
Quantitative analysis of anticancer 3-deoxyanthocyanidins in...
2007-01-24 [J. Agric. Food Chem. 55(2) , 254-9, (2007)] |
|
Antioxidant capacity of a 3-deoxyanthocyanidin from soybean.
2001-12-01 [Phytochemistry 58(7) , 1097-105, (2001)] |