Discovery, biological evaluation, and structure-activity and -selectivity relationships of 6'-substituted (E)-2-(benzofuran-3(2H)-ylidene)-N-methylacetamides, a novel class of potent and selective monoamine oxidase inhibitors.
Leonardo Pisani, Maria Barletta, Ramon Soto-Otero, Orazio Nicolotti, Estefania Mendez-Alvarez, Marco Catto, Antonellina Introcaso, Angela Stefanachi, Saverio Cellamare, Cosimo Altomare, Angelo Carotti
文献索引:J. Med. Chem. 56(6) , 2651-64, (2013)
全文:HTML全文
摘要
The use of selective inhibitors of monoamine oxidase A (MAO-A) and B (MAO-B) holds a therapeutic relevance in the treatment of depressive disorders and Parkinson's disease (PD), respectively. Here, the discovery of a new class of compounds acting as monoamine oxidase inhibitors (MAO-Is) and bearing a 6'-substituted (E)-2-(benzofuran-3(2H)-ylidene)-N-alkylacetamide skeleton is reported. 6'-Sulfonyloxy derivatives exhibited outstanding affinities to MAO-A (7.0 nM < IC50 < 49 nM, much higher than moclobemide) and a pronounced MAO-A/B selectivity. The corresponding 6'-benzyloxy derivatives showed potent MAO-B inhibition and inverted selectivity profile. The rigid E-geometry of the exocyclic double bond allowed a more efficient binding conformation compared to more flexible and less active 2-(1-benzofuran-3-yl)-N-methylacetamide isomers and 4-N-methylcarboxamidomethylcoumarin analogues. Focused structural modifications and docking simulations enabled the identification of key molecular determinants for high affinity toward both MAO isoforms. These novel MAO-Is may represent promising hits for the development of safer therapeutic agents with a potential against depression, PD, and other age-related neurodegenerative pathologies.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
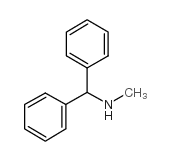 |
N-(二苯甲基)甲胺
CAS:14683-47-7 |
C14H15N |
|
Quantification of plasma HIV RNA using chemically engineered...
2014-01-01 [Nat. Commun. 5 , 5079, (2014)] |
|
Design, synthesis and characterisation of mannosylated ovalb...
2014-06-01 [Drug Deliv Transl Res 4(3) , 246-55, (2014)] |
|
Label-free measurement of histone lysine methyltransferases ...
2014-07-01 [Anal. Biochem. 456 , 25-31, (2014)] |
|
N-Diphenylmethyl-2-propenamide: theoretical study of ...
[J. Mol. Struct. 572(1) , 113-19, (2001)] |
|
An Improved Iron-Catalyzed Epoxidation of Aromatic and Aliph...
[European J. Org. Chem. 29 , 4867-70, (2008)] |