Oxidative metabolism of cinnarizine in rat liver microsomes.
S Kariya, S Isozaki, S Narimatsu, T Suzuki
文献索引:Biochem. Pharmacol. 44(7) , 1471-4, (1992)
全文:HTML全文
摘要
The oxidative metabolism of cinnarizine (CZ) [1-(diphenylmethyl)-4-(3-phenyl-2-propenyl)-piperazine] to 1-(diphenylmethyl)piperazine (M-1), 1-(diphenylmethyl)-4-[3-(4'-hydroxyphenyl)-2-propenyl]piperazine (M-2), benzophenone (M-3) and 1-[4'-hydroxyphenyl)-phenylmethyl]-4-(3- phenyl-2-propenyl)piperazine (M-4) has been studied in rat liver microsomes. In Wistar rats, kinetic analysis revealed sex differences (male > female) in the Km values for formation of all the metabolites and the Vmax values for the formation of M-1, M-3 and M-4. The reactions required NADPH, and were inhibited by carbon monoxide and SKF 525-A. Only M-2 formation was suppressed by sparteine or metoprolol, and was significantly lower in female Dark Agouti rats than in Wistar rats of both sexes. The results suggest that CZ is oxidized by cytochrome P450, and M-2 formation is related to debrisoquine/sparteine-type polymorphic drug oxidation.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
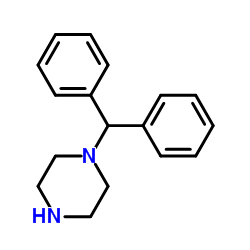 |
二苯甲基哌嗪
CAS:841-77-0 |
C17H20N2 |
|
Quantification of cyclizine and norcyclizine in human plasma...
2011-03-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(9-10) , 605-9, (2011)] |
|
The reactions of polyhalogenated-2-nitro-1, 3-butadiene with...
[Indian J. Chem. B 47(9) , 1407, (2008)] |
|
Functionalization of sterically hindered o-benzoquinones: am...
[Russ. Chem. Bull. 56(9) , 1849-56, (2007)] |
|
Synthesis and in vitro antiproliferative activity of novel 1...
2009-03-01 [Eur. J. Med. Chem. 44(3) , 1223-9, (2009)] |
|
The pharmacokinetics and pharmacogenetics of the antiemetic ...
2012-03-01 [J. Pain Symptom Manage. 43(3) , 540-8, (2012)] |