Structure of lactate dehydrogenase from Plasmodium vivax: complexes with NADH and APADH.
Apirat Chaikuad, Victoria Fairweather, Rebecca Conners, Tim Joseph-Horne, Dilek Turgut-Balik, R Leo Brady
文献索引:Biochemistry 44(49) , 16221-8, (2005)
全文:HTML全文
摘要
Malaria caused by Plasmodium vivax is a major cause of global morbidity and, in rare cases, mortality. Lactate dehydrogenase is an essential Plasmodium protein and, therefore, a potential antimalarial drug target. Ideally, drugs directed against this target would be effective against both major species of Plasmodium, P. falciparum and P. vivax. In this study, the crystal structure of the lactate dehydrogenase protein from P. vivax has been solved and is compared to the equivalent structure from the P. falciparum enzyme. The active sites and cofactor binding pockets of both enzymes are found to be highly similar and differentiate these enzymes from their human counterparts. These structures suggest effective inhibition of both enzymes should be readily achievable with a common inhibitor. The crystal structures of both enzymes have also been solved in complex with the synthetic cofactor APADH. The unusual cofactor binding site in these Plasmodium enzymes is found to readily accommodate both NADH and APADH, explaining why the Plasmodium enzymes retain enzymatic activity in the presence of this synthetic cofactor.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
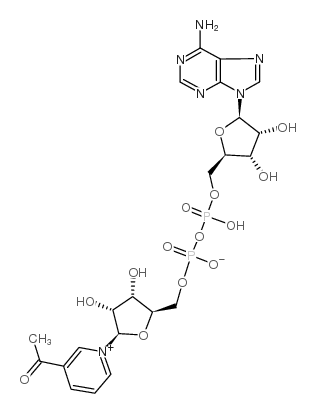 |
3-乙酰吡啶腺嘌呤二核苷酸
CAS:86-08-8 |
C22H28N6O14P2 |
|
Investigation of NADH binding, hydride transfer, and NAD(+) ...
2013-06-11 [Biochemistry 52(23) , 4048-55, (2013)] |
|
Nowhere to hide: interrogating different metabolic parameter...
2015-01-01 [Malaria Journal 14 , 213, (2015)] |
|
A combination of new screening assays for prioritization of ...
2015-05-01 [J. Antimicrob. Chemother. 70 , 1357-66, (2015)] |
|
Transhydrogenation reactions catalyzed by mitochondrial NADH...
2007-12-11 [Biochemistry 46(49) , 14250-8, (2007)] |
|
Protein engineering tests of a homology model of Plasmodium ...
1997-01-01 [Protein Eng. 10(1) , 39-44, (1997)] |