Conformational analysis of homochiral and heterochiral diprolines as beta-turn-forming peptidomimetics: unsubstituted and substituted models.
P W Baures, W H Ojala, W B Gleason, R L Johnson
文献索引:J. Pept. Res. 50(1) , 1-13, (1997)
全文:HTML全文
摘要
The effect of replacing one of the proline residues in either unsubstituted homochiral or heterochiral diproline segments with either a 2- or a 3-substituted prolyl residue on the allowed conformational of the diproline template has been examined. In heterochiral (L-D) diprolines, placement of a 2-methyl-D-proline residue in the i + 2 position and placement of either a cis- or trans-3-methyl-L-proline residue in the i + 1 position results in substituted diproline peptides that adopt the same type II beta-turn conformation as that defined experimentally for the unsubstituted diproline peptides. In contrast, placement of a cis-3-methyl-D-proline residue in the i + 1 position of a homochiral (D-D) diproline peptide seems to promote a different conformation than that seen in the unsubstituted case, whereas the trans-3-methyl-D-proline residue seems to provide a stabilizing influence for the predicted type VI' beta-turn. The demonstrated ability of certain substituted diproline templates to adopt predictable conformations coupled with the development of asymmetric synthetic routes to both 2- and 3-substituted prolyl residues, capable of mimicking a variety of side chains should make these templates useful tools in designing specific turn mimics of biologically active molecules.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
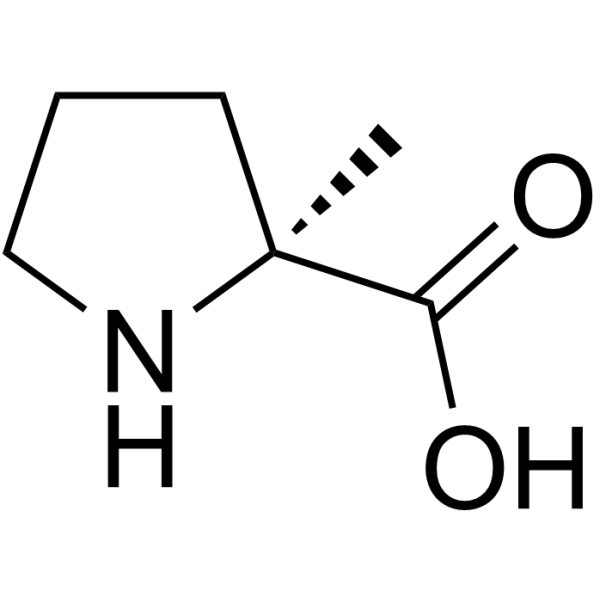 |
(S)-2-甲基脯氨酸
CAS:42856-71-3 |
C6H11NO2 |
|
Effects of ring contraction on the conformational preference...
2012-01-01 [Biopolymers 98(2) , 98-110, (2012)] |
|
Beta-turns induced in bradykinin by (S)-alpha-methylproline.
1992-02-10 [FEBS Lett. 297(3) , 216-20, (1992)] |
|
alpha-Methylproline-containing renin inhibitory peptides: in...
1987-03-01 [J. Med. Chem. 30(3) , 536-41, (1987)] |
|
Alpha-methyl-proline restores normal levels of bone collagen...
1995-01-01 [Life Sci. 57(24) , 2245-52, (1995)] |
|
Is the backbone conformation of C(alpha)-methyl proline rest...
2009-08-10 [Chemistry 15(32) , 8015-25, (2009)] |