Kinetic mechanism and ATP-binding site reactivity of p38gamma MAP kinase.
T Fox, M J Fitzgibbon, M A Fleming, H M Hsiao, C L Brummel, M S Su
文献索引:FEBS Lett. 461(3) , 323-8, (1999)
全文:HTML全文
摘要
Activated p38gamma MAP kinase exhibited significant basal ATPase activity in the absence of a kinase substrate, and addition of a phosphoacceptor substrate increased k(cat)/K(m)20-fold. AMP-PCP was competitive with ATP binding and non-competitive with phosphoacceptor substrate binding. The nucleotide binding site affinity label 5'-(p-fluorosulfonylbenzoyl)adenosine (FSBA) bound stoichiometrically at Lys-56 in the ATP site of both unphosphorylated and activated p38gamma. AMP-PCP only protected the activated enzyme from FSBA inactivation, implying that AMP-PCP does not bind unphosphorylated p38gamma. Basal ATPase activities were also observed for activated p38alpha, ERK2 and JNK3 suggesting that the enzymatic mechanism may be similar for all classes of MAP kinases.
相关化合物
| 结构式 | 名称/CAS号 | 分子式 | 全部文献 |
|---|---|---|---|
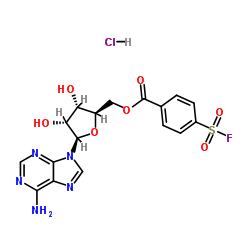 |
5ˊ-对氟磺酰苯甲酰腺苷盐酸盐
CAS:78859-42-4 |
C17H17ClFN5O7S |
|
Radioresistant Sf9 insect cells undergo an atypical form of ...
2013-12-01 [Int. J. Radiat. Biol. 89(12) , 1017-27, (2013)] |
|
Wortmannin inactivates phosphoinositide 3-kinase by covalent...
1996-04-01 [Mol. Cell. Biol. 16 , 1722-1733, (1996)] |
|
Vesicular transport and apotransferrin in intestinal iron ab...
2006-02-01 [Am. J. Physiol. Gastrointest. Liver Physiol. 290 , G301-G309, (2006)] |
|
The ATP-binding site of brain phosphatidylinositol 4-kinase ...
2001-03-01 [Int. J. Biochem. Cell Biol. 33(3) , 249-59, (2001)] |
|
5'-p-Fluorosulfonylbenzoyl adenosine inhibits progesterone s...
2002-11-08 [Biochim. Biophys. Acta 1585(1) , 11-8, (2002)] |